Villacorta Linaza, Rocio, Genovezos, Chantelle, Garner, Timothy, Panford-Quainoo, Edwin and Roberts, Adam  ORCID: https://orcid.org/0000-0002-0760-3088
(2022)
'Global antimicrobial stewardship and the need for pharmaceutical system strengthening for antimicrobials within a One Health approach'. International Journal of Pharmacy Practice, Vol 30, Issue 2, pp. 175-179.
ORCID: https://orcid.org/0000-0002-0760-3088
(2022)
'Global antimicrobial stewardship and the need for pharmaceutical system strengthening for antimicrobials within a One Health approach'. International Journal of Pharmacy Practice, Vol 30, Issue 2, pp. 175-179.
![[img]](https://archive.lstmed.ac.uk/19883/2.hassmallThumbnailVersion/Figure%201_JPEG.jpeg)
|
Image
Figure 1_JPEG.jpeg - Supplemental Material Download (77kB) | Preview |
|
![[img]](https://archive.lstmed.ac.uk/19883/3.hassmallThumbnailVersion/Figure%202_JPEG.jpeg)
|
Image
Figure 2_JPEG.jpeg - Supplemental Material Download (110kB) | Preview |
|
|
Text
Int_J_Pharm_Pract_ria012.pdf - Accepted Version Download (153kB) | Preview |
Abstract
Objectives
The Covid-19 pandemic has highlighted both the vulnerabilities and the critical role of global pharmaceutical systems in enabling equitable access to medicines. In this personal view, we position the pharmaceutical system as a missed research and investment opportunity that, if integrated properly, would benefit antimicrobial stewardship (AMS) programmes within a One Health approach.
Key findings
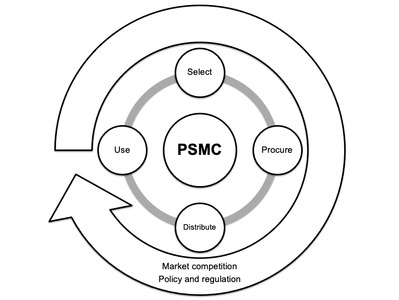
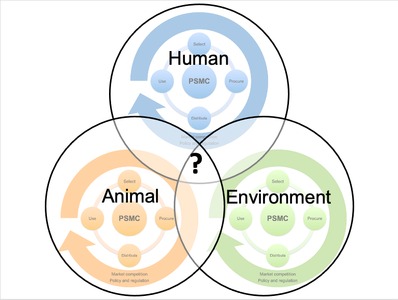
The pharmaceutical supply management cycle (PSMC) illustrates the continuous interdependence between four key phases - selection, procurement, distribution and use. Furthermore, a PSMC is subject to external forces of market competition, policy and regulation - across human, animal and environmental health. We present examples of overlap in PSMCs across different One Health sectors and discuss the need for integration within human, animal and environmental health contexts.
Summary
Despite pharmaceutical systems being fundamental to successful AMS programmes they are currently neglected and undervalued. Research and investment into pharmaceutical system optimisation and integration into AMS programmes presents an opportunity for both high income countries and low- and middle-income countries, to develop responsible, comparable and international AMS innovations and interventions.
| Item Type: | Article |
|---|---|
| Subjects: | QV Pharmacology > Drug Standardization. Pharmacognosy. Medicinal Plants > QV 771 Standardization and evaluation of drugs QW Microbiology and Immunology > QW 45 Microbial drug resistance. General or not elsewhere classified. WA Public Health > WA 30.7 One Health WA Public Health > Health Administration and Organization > WA 530 International health administration |
| Faculty: Department: | Biological Sciences > Department of Tropical Disease Biology |
| Digital Object Identifer (DOI): | https://doi.org/10.1093/ijpp/riac012 |
| Depositing User: | Marie Hatton |
| Date Deposited: | 29 Mar 2022 12:26 |
| Last Modified: | 08 Oct 2024 12:27 |
| URI: | https://archive.lstmed.ac.uk/id/eprint/19883 |
Statistics
Actions (login required)
 |
Edit Item |

 Tools
Tools Tools
Tools